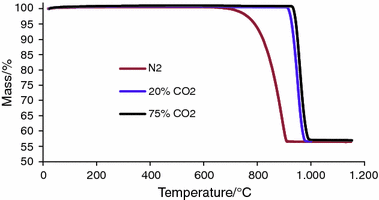
Applied Sciences | Free Full-Text | Comparative Kinetic Analysis of CaCO3/CaO Reaction System for Energy Storage and Carbon Capture

Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction - ScienceDirect
Mortar is made using limestone, which is composed primarily of calcium... | Download Scientific Diagram

Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction - ScienceDirect

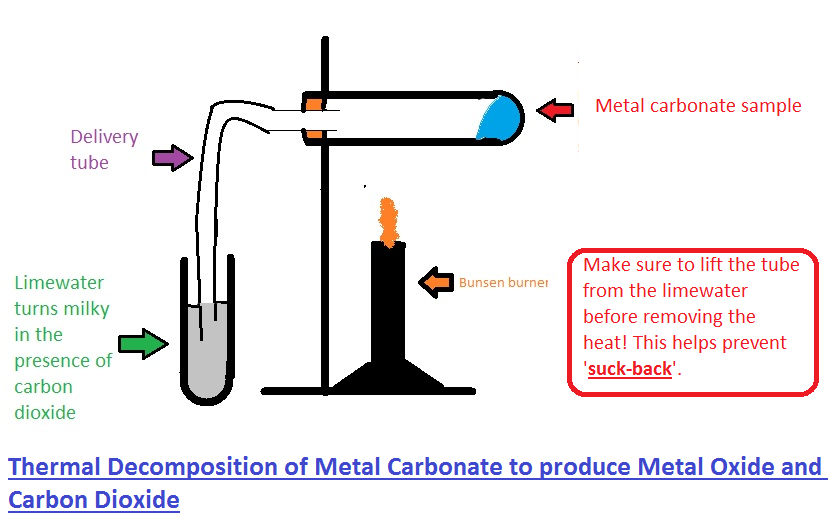
Draw the arrangement for heating of calcium carbonate and testing the gas evolved with burning match - Brainly.in

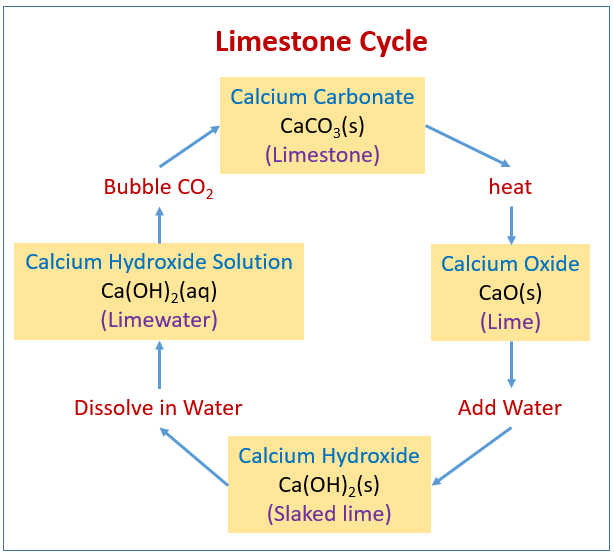
Carbon Dioxide from Thermal Decomposition (2.3.4) | Edexcel IGCSE Chemistry Revision Notes 2019 | Save My Exams
Write balanced chemical equation for the following processes: (a) heating calcium in oxygen (b) heating calcium carbonate - Sarthaks eConnect | Largest Online Education Community

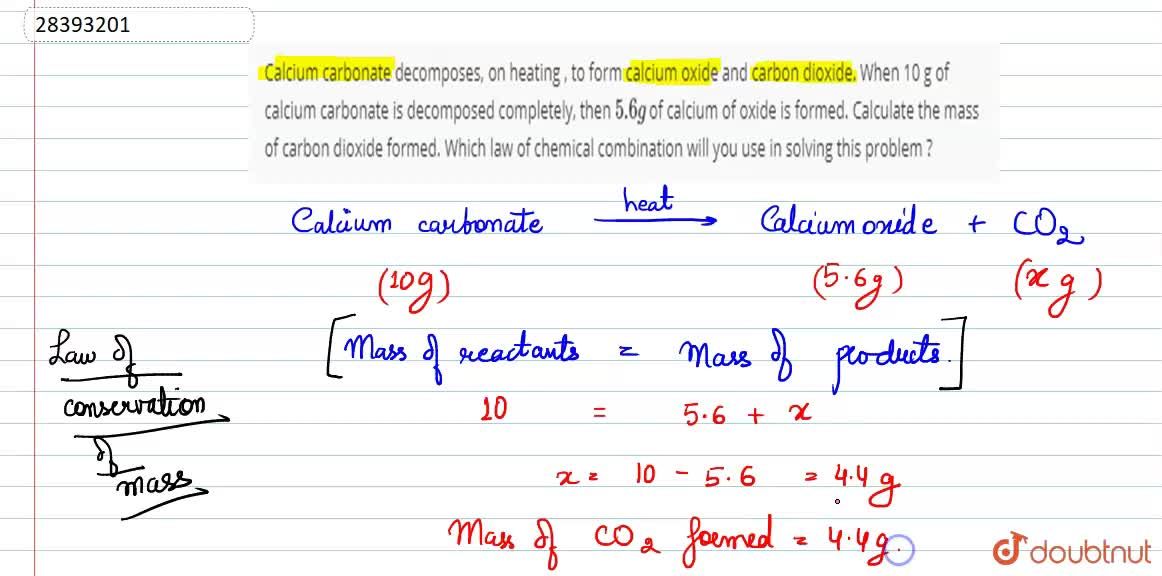
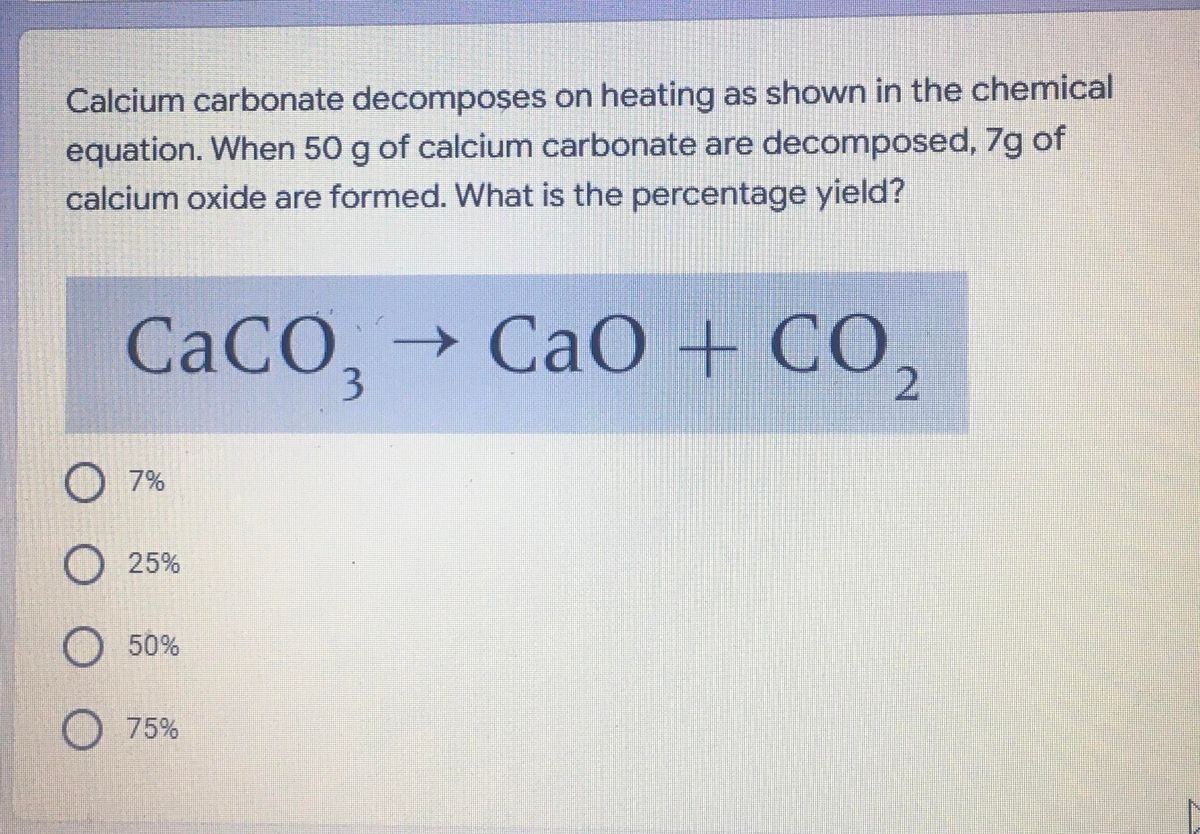
50 g of an impure calcium carbonate sample decomposes on heating to give carbon dioxide and 22.4 g calcium oxide. The percentage purity of calcium carbonate in the sample is:

Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa